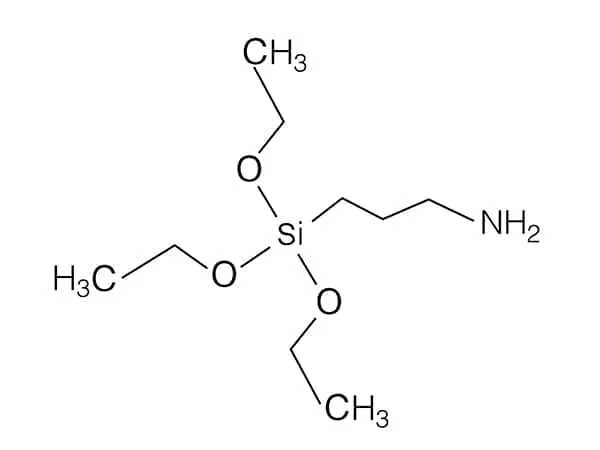
N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane is a specific organosilane coupling agent that contains both amino (-NH₂) and alkoxysilane (-Si(OR)₂CH₃) groups in its structure.
Chemical Structure: The molecule consists of:
Aminoethyl group (-CH₂CH₂NH₂): Provides primary amine functionality, which is reactive towards various substrates.
Aminopropyl group (-CH₂CH₂CH₂NH₂): Adds additional amine functionality, enhancing its reactivity.
Methyldimethoxysilane group (-Si(OCH₃)₂CH₃): This group is hydrolysable, allowing it to bond strongly to inorganic surfaces.
Functionality: N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane acts as a coupling agent and surface modifier:
Adhesion Promotion: Enhances bonding between organic materials (like polymers) and inorganic surfaces such as glass, metals, and silica.
Surface Modification: Forms a stable, covalent bond with substrates through the silane group, which can be further functionalized or coated.
Cross-Linking: Can participate in cross-linking reactions, contributing to the formation of networks in polymers and composites.
Applications:
Coatings and Adhesives: Used to improve adhesion strength and durability in paints, coatings, and adhesives.
Fillers and Composites: Enhances compatibility and dispersion of fillers (e.g., silica, glass fibers) in polymer matrices.
Bioconjugation: Functionalized surfaces are used in biochemistry and medical devices for immobilizing biomolecules.
Catalysis: Modified surfaces can serve as supports for catalysts in various chemical processes.
Hydrolysis: Like other silane coupling agents, N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane undergoes hydrolysis upon exposure to moisture. The methoxy groups (-OCH₃) are replaced by silanol groups (-OH), which can then condense with surface hydroxyl groups to form stable siloxane bonds.
Benefits: This silane coupling agent offers versatility, reactivity, and stability, making it valuable for modifying surfaces, enhancing adhesion, and tailoring interfaces in industrial and research applications.
In summary, N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane plays a crucial role in improving the performance and functionality of materials by facilitating strong bonds between organic and inorganic components, thereby enhancing overall properties and applications in various industries.
N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane is a specific organosilane coupling agent that contains both amino (-NH₂) and alkoxysilane (-Si(OR)₂CH₃) groups in its structure.
The molecule consists of:
Aminoethyl group (-CH₂CH₂NH₂): Provides primary amine functionality, which is reactive towards various substrates.
Aminopropyl group (-CH₂CH₂CH₂NH₂): Adds additional amine functionality, enhancing its reactivity.
Methyldimethoxysilane group (-Si(OCH₃)₂CH₃): This group is hydrolysable, allowing it to bond strongly to inorganic surfaces.
Hydrolysis: Like other silane coupling agents, N-(2-Aminoethyl)-3-aminopropylmethyldimethoxysilane undergoes hydrolysis upon exposure to moisture. The methoxy groups (-OCH₃) are replaced by silanol groups (-OH), which can then condense with surface hydroxyl groups to form stable siloxane bonds.