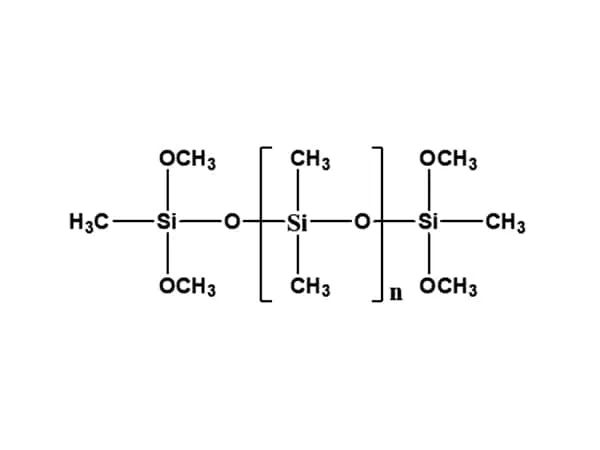
Silane is the silicon analog of methane in which a hydrogen is substituted by an organic functional group such as vinyl, amino, chloro, epoxy, mercapto, alkoxy, etc. Usually, silanes possess a hydrolytically sensitive center (e.g. –OR) that can react with hydroxyls of inorganic substrates to form stable covalent bonds, thus providing the inorganics with organic tails (e.g. R) that act as coupling agents between inorganic particles and polymer matrices. Silanes are used in fire-retardant materials taking advantage of the fairly good thermal stability and the coupling action to link inorganic flame retardants and polymer matrixes. While obtaining a good balance of strength and toughness [10], improved fire-retardant properties is obtained thanks to the improved dispersion of fire-retardant particles in the polymer [11] and due to the enhanced interaction between fire-retardant components [12], possibly leading to enhanced stability of combustion residues acting as a thermal shield toward the flame [13]. An active fire-retardant role is played by silanes which bear reactive functional groups that enable them to be incorporated in the polymer structure. Examples of silanes that have been reported in literature as fire retardants in different polymers are mentioned